Campus News
UC Santa Cruz scientists reveal new path to increasing lactation for nursing mothers
Scientists at UC Santa Cruz have discovered a cellular process in the breast that can increase milk production by pregnant women, revealing a potential path to addressing lactation insufficiency syndrome—the inability of a nursing mother to produce sufficient milk to meet their infant’s nutritional needs.

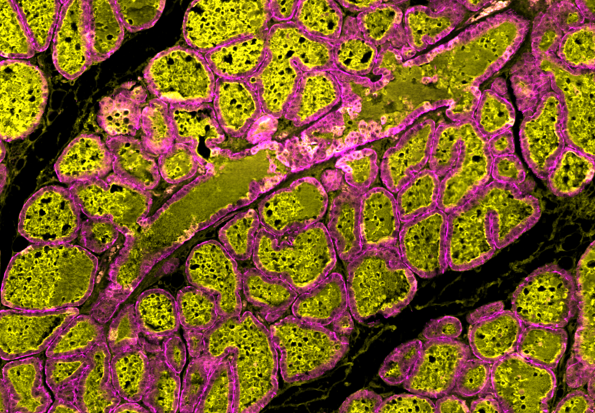
Scientists at UC Santa Cruz have discovered a cellular process in the breast that can increase milk production by pregnant women, revealing a potential path to addressing lactation insufficiency syndrome—the inability of a nursing mother to produce sufficient milk to meet their infant’s nutritional needs.
The new study, published on April 17 in Nature Communications, identifies an enzyme that promotes the generation of more milk-producing cells by halting cell division at just the right time. This enzyme, called WEE1, is activated by the cell as a natural response to the damaging effects of rapid DNA replication, which is required during pregnancy to build a milk supply.
Previous studies have shown that a large proportion of the milk-producing alveolar cells in the breast contain two nuclei at the onset of lactation. The presence of these cells is integral for efficient milk production, but it was thought that chance dictated how many of these bi-nucleated cells would be generated.
This new study, however, found that the WEE1 enzyme actively stops cell division after the genome is replicated, but before the cell itself divides. This process—known as endoreplication—generates cells that contain more than the normal complement of DNA, which is two copies of each chromosome. The result is a “polyploid” cell with two nuclei, each with the normal complement of DNA—or even a single nuclei with double the normal complement. Furthermore, this can be repeated many times to generate cells with even more DNA.
Understanding this process presents new possibilities for treatments aimed at increasing the proportion of polyploid alveolar cells, and hence, increasing milk production. A mother’s milk is “liquid gold,” said UC Santa Cruz professor Lindsay Hinck. “Breastfeeding confers a host of lifelong benefits to both mother and child, yet upwards of 50% of women worldwide experience lactation insufficiency.”
Hinck is a distinguished professor of molecular, cell and developmental biology, and the lead authors on the paper presenting these new findings are Rut Molineuvo and Julien Menendez—respectively, former and current postdoctoral fellows in Hinck’s lab. With UC Santa Cruz biomolecular engineering professor Camilla Forsberg, Hinck also directs the Institute for the Biology of Stem Cells (IBSC), which advances stem-cell research by promoting interdisciplinary discoveries in biology, engineering, and information science.
Low lactation is but one reason for breastfeeding problems; other challenges are maternal stress and infant latching issues. But Hinck points out that milk substitutes are popular, in part, because many women struggle to meet the American Academy of Pediatrics recommendation that infants be exclusively breastfed for the first six months. “The recent formula shortage in America underscores the reliance of many parents on mother’s milk substitutes,” Hinck said. “Our study offers insight into why women may experience the differential ability to produce sufficient milk during lactation.”
During pregnancy, cell division ramps up to generate the large number of cells required for milk production. This rapid cell division leads to replication errors—DNA damage—that is not corrected. Instead, the cell pauses in its division process and switches to an “endocycle” in which the DNA is doubled in the absence of cell division. Fortunately, endoreplication plays a regulatory role and halts cell division permanently, thereby preventing the endless growth associated with cancerous states.
“By unraveling the DNA damage-response signaling pathway regulating endoreplication, we’ve identified a non-hormonal approach to increase milk production and potential targets for therapeutic intervention that may ultimately aid women experiencing lactation insufficiency,” Hinck said. “We think our discovery is of broad interest, especially in light of new calls by the White House to invest in and advance women’s health research and innovation.”
The other authors on this paper, “Physiological DNA damage Promotes Functional Endoreplication of Mammary Gland Alveolar Cells During Lactation,” included researchers from UC Santa Cruz’s Department of Molecular, Cell, and Developmental Biology, IBSC, and Zoetis, Inc.
The study was funded by grants from the National Institutes of Health (R01HD098722 to Hinck and T32HD108079 to Menendez). Other support came from a California Institute of Regenerative Medicine training grant (EDUC-12759) and the National Institutes of Health’s Maximizing Access to Research Careers program.