Campus News
Cholera studies reveal mechanisms of biofilm formation and hyperinfectivity
A series of publications from microbiologist Fitnat Yildiz’s lab provides new understanding of cholera biofilms and why the bacteria in biofilms are so highly infectious.
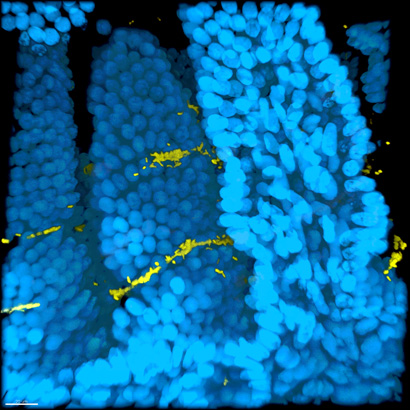
Imaris Snapshot

Free-swimming cholera bacteria are much less infectious than bacteria in biofilms, aggregates of bacterial cells embedded in a sticky matrix that form on surfaces. This accounts for the surprising effectiveness of filtering water through cloth, such as a folded sari, which can reduce infections dramatically in places where the disease is endemic, despite the fact that individual cholera bacteria easily pass through such a filter.
A new study led by researchers at UC Santa Cruz goes a long way toward explaining the hyperinfectivity of cholera biofilms. The study, published the week of April 20 in the Proceedings of the National Academy of Sciences (PNAS), is one of several new papers on cholera biofilms from the laboratory of UCSC microbiologist Fitnat Yildiz.
“We’ve been working on this for so long, it is a significant body of work that is now being published, focusing on the mechanisms of biofilm formation and what makes the biofilm more infectious,” said Yildiz, a professor of microbiology and environmental toxicology.
Biofilms are important not only in causing infections, but also in the survival of cholera bacteria (Vibrio cholerae) in the environment. In regions where cholera is endemic, the bacteria live in aquatic environments, typically in brackish water, causing periodic, seasonal outbreaks when sources of drinking water become contaminated.
A surprising finding in the PNAS paper is that bacteria growing in biofilms have already activated the genes for virulence factors such as toxin production, before they have even infected a host.
“Two of the main virulence factors are the toxin co-regulated pilus, which allows the bacteria to adhere to the intestine, and the cholera toxin which enters intestinal cells and makes people really sick,” said Jennifer Teschler, a postdoctoral researcher in the Yildiz lab and a co-first author of the paper. “These virulence factors are more highly expressed in biofilm cells, so they are already primed for causing infections.”
The study also showed differences in the colonization patterns of free-swimming (“planktonic”) and biofilm-grown cholera cells in the intestines of infected mice. The researchers used a new imaging technique to make intestinal tissue transparent while preserving the spatial integrity of the infected intestines. This enabled them to see where the cholera bacteria had adhered to the villi, the finger-like projections that line the small intestine.
“Being able to see where the infections are in three dimensions is an important tool for studying intestinal pathogens,” Teschler said. “In mice infected with planktonic cells, the cells were typically at the bottom of the villi, whereas biofilm cells attached at the top of the villi, closer to the lumen. We speculate that biofilm cells adhere more strongly to the villi, so they are better able to resist being swept away by the flow in the lumen of the intestine.”
Two other papers, published March 25 in Nature Communications and March 16 in PLOS Genetics, focus on how free-swimming cholera bacteria attach to surfaces and initiate biofilm formation.
“The bacterium has to attach to a surface, stop swimming, and start building a matrix,” Yildiz said. “Understanding the mechanisms involved in biofilm formation, as well as the role of biofilms in the overall biology of Vibrio cholerae, will pave the way for developing strategies to predict and control cholera epidemics. It may also help in identification of novel drug targets for inhibiting biofilm formation during infection.”
The Nature Communications paper explores the cellular signaling pathways that control the attachment process through the regulation of hair-like appendages called pili that grow out from the cell surface.
“Attachment is the initiating step of biofilm formation,” explained first author Kyle Floyd, a postdoctoral researcher in the Yildiz lab. “As a swimming cell nears a surface, the pilus will bind to the surface, and retraction of the pilus helps pull the cell closer to the surface. The cell then makes more pili to anchor it down to the surface.”
There are different classes and subclasses of bacterial pili, and the one required for biofilm formation in many Vibrio cholerae strains (the type IV MSHA pilus) is regulated by a signaling molecule called c-di-GMP. The new study showed that the MSHA pilus is a dynamic system that extends and retracts and is directly controlled by c-di-GMP. The study showed how pilus activity is modulated by the interactions of c-di-GMP with other components of the pilus system.
The PLOS Genetics paper further elucidates the c-di-GMP signaling pathways that promote biofilm formation. In particular, the study looked at the role of the flagellum, a whip-like appendage the bacteria use to swim, in c-di-GMP signaling. The researchers found that loss of the flagellum leads to elevated levels of c-di-GMP in the cell and increased expression of biofilm genes.
“It required powerful and elegant genetics to work out the connections between flagellum assembly, production of pili on the cell surface, biofilm matrix production, and c-di-GMP signaling,” Yildiz said. “There are different steps where this signaling molecule can control the transition to biofilm formation.”
All three papers involved extensive collaboration with researchers at other institutions. The coauthors of the PNAS paper, in addition to Yildiz and Teschler, include co-first authors Ana Gallego-Hernandez and Jinhwan Park at UCSC and William DePas at the California Institute of Technology; Raimo Hartmann, Hannah Jeckel, and Knut Drescher at the Max Planck Institute for Terrestrial Microbiology in Germany; Sinem Beyhan at the J. Craig Venter Institute; and Dianne Newman at Caltech. This work was funded by the National Institutes of Health.