The retinoblastoma tumor suppressor protein acts like a gate in the cycle of cell growth and division--a gate that stays open in many types of cancer, allowing cells to multiply out of control. New findings by cancer researchers at the University of California, Santa Cruz, reveal the molecular mechanisms involved in opening and closing that gate.
"We really need to understand how these proteins work at a mechanistic level if we're ever going to successfully target them with drugs," said Seth Rubin, assistant professor of chemistry and biochemistry at UC Santa Cruz. "The easiest type of drug to make is one that knocks out a protein function, but in this case you want the protein to function--you want it to be a gate that opens and closes at the right times."
The retinoblastoma protein was already known to be regulated by the addition and removal of phosphate groups. An enzyme called cyclin-dependent kinase adds phosphates (a process called phosphorylation), while another enzyme called protein phosphatase 1 removes them. When the retinoblastoma protein is phosphorylated, the gate is open and the cell cycle proceeds to cell division; the gate closes when the phosphates are removed.
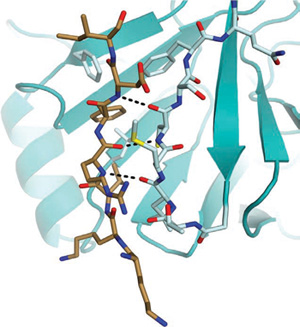
Rubin's team used x-ray crystallography to figure out the three-dimensional structure of the retinoblastoma protein with the phosphatase enzyme bound to it. To their surprise, they found that when the phosphatase attaches to the protein to close the gate, it binds to the same site known to be used by the kinase when it adds phosphates and opens the gate. So the two enzymes are competing for the same "docking site" needed to control the gate.
"It explains how the binding of the phosphatase not only acts to close the gate by removing phosphates, but then keeps the gate closed by blocking access to the docking site so that the kinase can't get on and open the gate," Rubin said.
The researchers reported their findings in the August 8 issue of Nature Structural & Molecular Biology. Alexander Hirschi, a graduate student in molecular, cell, and developmental biology, is the lead author of the paper. Coauthors include Rachel Steinhardt, a graduate student in chemistry and biochemistry, and Matthew Cecchini and Frederick Dick of the London Regional Cancer Program of the Children's Health Research Institute in Ontario, Canada.
Hirschi performed most of the crystallography work involved in determining the protein structure. The researchers then carried out biochemical experiments to show that the two enzymes actually do compete for the same binding site in order to carry out their respective functions. Finally, the UCSC group teamed up with Canadian cancer researchers Cecchini and Dick to perform experiments in living cells.
Laboratory studies in cultured cells confirmed that the regulation of cell growth and division involves competition between kinase and phosphatase for the binding site on the retinoblastoma protein. In one experiment, the researchers used a mutant version of phosphatase unable to perform its normal phosphate-removing function to show that simply by binding to the retinoblastoma protein it was able to block the action of the kinase.
Kinases are already key targets in drug discovery efforts. Understanding the competitive interplay between kinases and phosphatases may help guide those efforts, Rubin said. "This kind of research is necessary to develop a fundamental understanding of these key proteins and may lead to new ways of targeting them," he said.
This research was funded by the U.S. National Institutes of Health and the Canadian Institutes of Health Research. Rubin's lab also receives funding from the Santa Cruz Cancer Benefit Group, a local charity supporting cancer research and patient care.